Abstract
Introduction: Success of T cell therapy is restricted by the variable success of expanding patients' T cells ex vivo and the limited persistence of cultured T cells in T cell-treated patients. These problems may be particularly relevant to lymphoma patients previously treated with numerous cycles of chemotherapy, and motivate the development of novel strategies to enhance ex vivo T cell expansion and their persistence in vivo . Recently we have shown that antagonism of vasoactive intestinal peptide (VIP) signaling on lymphocytes augments in vivo T cell activation and growth leading to significant and durable T cell-dependent anti-cancer immune response in murine models of lymphoma and leukemia (Li Cancer Research 2016; Petersen Oncoimmunology 2017). We tested the hypothesis that VIP antagonists would synergize with phosphatidyl inositol-3 kinase inhibitors (PI3Ki) to augment T-cells ex vivo expansion of T cells with anti-cancer immune functions.
Methods: Peripheral blood mononuclear cells were obtained from consenting DLBCL patients and healthy controls. Frozen PBMC were thawed and rested overnight in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol then cultured in 96 well flat-bottom plates with 30 U/mL IL-2 and anti-CD3/CD28 beads at a 1:1 bead to cell ratio in the presence of idelalisib, duvelisib, a peptide antagonist to VIP (VIPhyb, KPRRPYTDNYRELRKQMAVKKYLNSILN), or mast cell chymase, a VIP-degrading enzyme. Flow cytometric analysis of T cells from patients, healthy volunteers, and mice prior to and following ex vivo cell expansion measured expression of CD3, CD4, CD8, CD27, CD28 and intracellular cytokines (IL2, IFN-gamma). In vivo persistence of cultured human T -cells was monitored in following ex vivo expansion and transfer into RAG mice. Anti-lymphoma activity of cultured murine T cells was assessed by culturing a mixture of wild-type, OT1, and OT2 murine T-cells and transfer into immune-competent mice bearing subcutaneous E.G7 OVA murine B cell lymphoma.
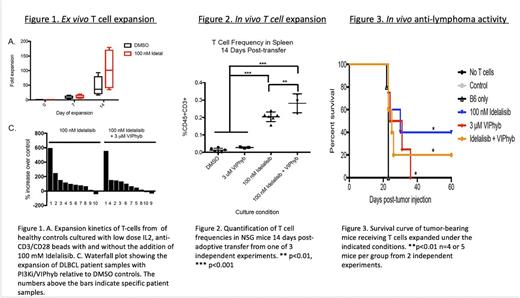
Results: T cells from heavily treated DLBCL patients had a senescent phenotype with loss of CD27 and CD28 expression, with a median of 36.8% (range 4.58-77.3) CD27-CD28- T cells compared with median values of 5.8%, range 1.7-12.4% in healthy controls and 3.9%, range 0.5-23.1% in untreated DLBCL patients. FACS-purified senescent CD27- CD28- T cells failed to expand ex vivo and had fratricide-mediated inhibition of non-senescent T cells expansion. Blockade of PI3K δ and antagonism of vasoactive intestinal peptide (VIP) signaling during T-cell culture with low dose IL2 and anti- CD3/CD28 beads partially inhibited terminal differentiation (mean frequency 54.4% CD27+CD28+ T cells vs 27.4% in control cultures, p<0.05) during anti-CD3/CD28 bead-mediated expansion. This culture strategy increased yields of T cells cultured from lymphoma patients with a median of 83.7% more T cells when expanded in the presence of PI3K δ and VIP antagonists (Figure 1), and increased survival of human T cells from lymphoma patients in a murine xenograft model (Figure 2). Expanded T cells expressed multiple Th1 cytokines. Similar effects on T cell expansion were seen with the combination of PI3Ki and mast cell chymase (not shown). To test the anti-tumor activity of VIPhyb/PI3Ki expanded T cells in vivo CD45 congenic B6 SJL mice were subcutaneously injected with OVA-expressing E.G7 lymphoma cells in the right flank. Tumors were allowed to grow for seven days upon which a mixture of ex vivo -expanded OT-I (CD8), OT-II (CD4), and non-specific B6 T cells (in a 2:1:2 ratio; 5 x 106 total T cells) were injected intravenously. Murine OT1/OT2 T-cells expanded with the combination of PI3Ki/VIPhyb had enhanced cytotoxic anti-lymphoma activity in mice with OVA+ lymphoma (Figure 3). The PI3K δ and VIP antagonist-expanded T cells from lymphoma patients had increased expression of the anti-apoptotic Bcl-2 protein, reduced terminal differentiation of T cells, and preservation of co-stimulatory molecule expression.
Discussion: Taken together, these data demonstrate that synergistic blockade of the PI3K and VIP pathways is an attractive strategy to enhance the expansion and functional capacity of T cells during clinical manufacturing, and that optimization of culture expansion conditions can improve the magnitude and durability of anti-lymphoma responses in vivo .
Waller: Coulter Foundation: Research Funding; PRA: Consultancy; Chimerix: Equity Ownership; Katz Foundation: Research Funding; Celldex: Consultancy; Helocyte: Consultancy; AMGEN: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Cambium Medical Technologies: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Cerus: Equity Ownership; National Institutes of Health: Research Funding. Jagirdar: Kite: Consultancy. Flowers: Research to Practice: Research Funding; Burroughs Welcome Fund: Research Funding; National Institutes Of Health: Research Funding; Educational Concepts: Research Funding; Prime Oncology: Research Funding; Acerta: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Spectrum: Consultancy; Seattle Genetics: Consultancy; Millennium/Takeda: Research Funding; Genentech/Roche: Consultancy, Research Funding; Eastern Cooperative Oncology Group: Research Funding; OptumRx: Consultancy; TG Therapeutics: Research Funding; Infinity: Research Funding; Onyx: Research Funding; Clinical Care Options: Research Funding; Janssen Pharmaceutical: Research Funding; National Cancer Institute: Research Funding; Celgene: Consultancy, Research Funding; V Foundation: Research Funding; Gilead: Consultancy; Bayer: Consultancy; Abbvie: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal